Our Location
Understanding the melting points of different metals is crucial, particularly if you work in metal fabrication or manufacturing. A metal’s melting point is the temperature or heat level at which its solid structure changes into a molten or liquid form. This blog provides valuable insights into the significance of melting points, the melting points of common metals, scientific techniques for measurement, factors that affect metal melting points, and applications.

Table of Contents
ToggleThe melting point is the temperature at which a substance shifts from solid to liquid at atmospheric pressure. At this point, both solid and liquid states exist in equilibrium. It is dependent on pressure and usually specified at standard pressure in reference materials. It is also known as the liquefaction point, liquidus, or solidus.
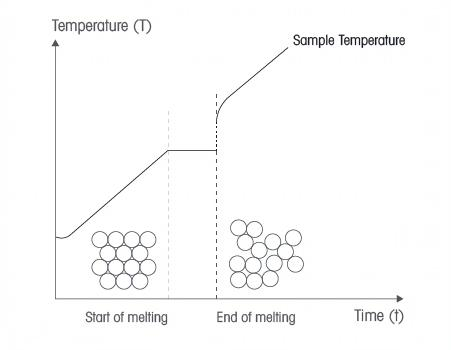
Accounting for a metal’s melting temperature is crucial to avoid fatal failure due to softening, which reduces material strength before reaching the melting point.
Below are some significant metal sectors and their corresponding melting points.
Melting points of metals aid their identification, classification, and alloy composition analysis.
In manufacturing, knowing the melting points of metals guides setting processing temperatures.
If you are a metal fabricator, you can use the melting points of metals as a measure of quality control. If you apply excessive heat during processing, you can note the excess temperature and compare it with the standard melting point. This information can then be used to determine if the overheating has caused any damage to the metal.
Understanding the melting points of different metals is crucial to choosing the suitable material for your application. Opt for metals with high melting points for high-temperature environments like a furnace. Also, remember that the larger and thicker your metal design, the longer it takes to melt completely.
The melting point of a metal determines its production process and application area. Here is a list of common metals in the manufacturing industry:
| Metals | Celsius(c) | Fahrenheit (f) |
| Aluminum | 660 | 1220 |
| Aluminum Bronze* | 1027-1038 | 1881-1900 |
| Brass | 930 | 1710 |
| Copper | 1084 | 1983 |
| Chromium | 1860 | 3380 |
| Gold | 1063 | 1945 |
| Inconel* | 1390-1425 | 2540-2600 |
| Cast Iron | 1204 | 2200 |
| Lead | 328 | 622 |
| Molybdenum | 2620 | 4748 |
| Nickel | 1453 | 2647 |
| Platinum | 1770 | 3218 |
| Silver | 961 | 1762 |
| Carbon Steel* | 1425-1540 | 2597-2800 |
| Stainless Steel* | 1375 – 1530 | 2500-2785 |
| Titanium | 1670 | 3038 |
| Tungsten | 3400 | 6152 |
| Zinc | 420 | 787 |
| **Alloys have a range of melting temperatures depending on their composition, which consists of more than one element. | ||
The melting point of a metal refers to the temperature at which it starts to shift from a solid state to a liquid state. However, this temperature is not constant under all conditions. The melting points of pure metals are affected by various factors, such as:
Metals have delocalized valence electrons that move freely throughout their lattice structure to create metallic solid bonds between atoms. The more delocalized electrons a metal has, the stronger the bonds and the higher the melting point.
Metallic elements are composed of atoms that have a distinct number of protons and electrons. The outer shells of these atoms are occupied by electrons, which enable the atoms to bond with one another and create metallic lattice structures. As a result, the more electrons a metal atom contains, the more robust the bonds between the atoms become, which leads to a higher melting point for the metal.
An element’s atomic mass is determined by its protons and neutrons. The higher the atomic mass, the more electrons the metal has on its outer shell. Electrons facilitate bonding between atoms, so the more energy required to break the bond, the higher the melting point of the metal.
Metals with larger grain sizes have higher melting points due to increased atom bonding.
Metals can exist in a crystalline or amorphous structure in its solid state. The melting point of a metal is determined by its crystalline structure.
Heating a metal increases the energy of its atoms, causing them to vibrate and weaken their forces of attraction. As the temperature rises, the atoms acquire enough energy to break free, causing the metal to melt.
Impurities can weaken the bonds in metal’s lattice structure during heating, decreasing melting point.
A pure metal has a uniform atomic structure with strong bonds between the atoms. However, the atomic bonds are weakened when different elements of varying sizes are added to create an alloy. This results in alloys having lower melting points than the individual metals that make them up.
Increasing pressure has opposite effects on the melting points of different substances. For example, while increasing pressure lowers the melting point of ice, increasing pressure increases the melting point of metals.
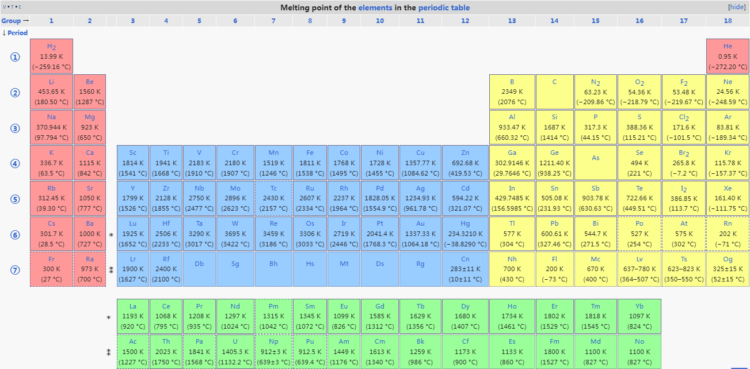
Source: Melting point of the elements
If you need to measure the melting point of metals for quality control or research purposes, you can use several laboratory techniques.
Differential Scanning Calorimetry measures a metal’s melting point. The technique involves heating a metal sample in a controlled atmosphere, recording temperature changes, and analyzing the data. It is useful for thermally stable metals that can be heated at high temperatures.
Thermogravimetric measuring involves heating a metal sample in a controlled furnace and monitoring its change in mass over time or temperature changes using a sensitive weighing scale. Analyzing the data obtained from the scale can determine the metal’s melting point.
To measure a metal’s melting point, observe its changes as it’s heated under a microscope. The changes in its structure and shape reveal the melting point, and this method can detect even small temperature changes that other methods may miss.
Knowing metals’ melting points is crucial for extrusion, casting, and forging processes in metal manufacturing. Choose the right material and determine the correct melting temperatures to produce quality parts.
Knowing metals’ melting points is crucial in welding. It helps you choose the right material, welding technique, equipment, and temperature levels for strong, durable, and high-quality welds.
Aircraft metal parts are exposed to high pressure and temperature during flights. To withstand these conditions, parts need superior strength and melting temperatures. Titanium and its alloys are commonly used in aircraft manufacturing due to their excellent properties and high melting points.
As a metals researcher, understanding their melting points helps create new alloys for industry. Better boiling point knowledge opens up research in electronics, new energy, and medical fields.
Automotive metal parts have different melting points. Stainless steel is used for car exhaust systems due to its high melting point, while aluminum is used for an engine’s cylinder head because of its low melting point. Knowing the melting points of metals during parts production helps prevent overheating defects.
Why do metals have different melting points?
Metals’ melting points vary due to their unique atomic structures and bond strengths. Each metal has a distinct atomic arrangement and electron configuration that affect the strength of its metallic bonds. Metals with more delocalized electrons and closely packed atoms have stronger bonds that require more energy to break, resulting in higher melting points. Other factors like atomic size, electron delocalization, and lattice structure complexity also contribute to this variance. Additionally, impurities and alloys can affect a metal’s melting point.
Can the melting point of a metal vary depending on different circumstances?
A metal’s melting point is generally constant under normal conditions. However, high pressure can increase it, and reduced dimensions can decrease it. Impurities and alloying elements can also alter the melting point. However, a pure metal’s melting point remains constant in everyday conditions.
Which Metal Has the Highest and the Lowest Melting Point?
Nickel and tungsten have very high melting temperatures at the highest end of the melting point range. Nickel’s melting point is around 1,452 °C/2,646 °F, while tungsten’s is around 3,399 °C/6,150°F.
On the other hand, at the lower end of the melting point spectrum, lead has a relatively low melting temperature of 327 °C/621 °F.
However, Metals don’t melt at just one temperature; instead, they have a range of temperatures called the Solidus to Liquidus range. This range goes from when the metal is solid to when it becomes liquid.
Metal melting point is the energy needed to transform a solid metal into a liquid. This knowledge is crucial for identifying and classifying metals. Understanding melting points helps determine suitable temperatures for manufacturing processes, ensuring products fulfill their applications. You can measure melting points using lab techniques for quality control or research. Contact us if you have more questions.

