Our Location
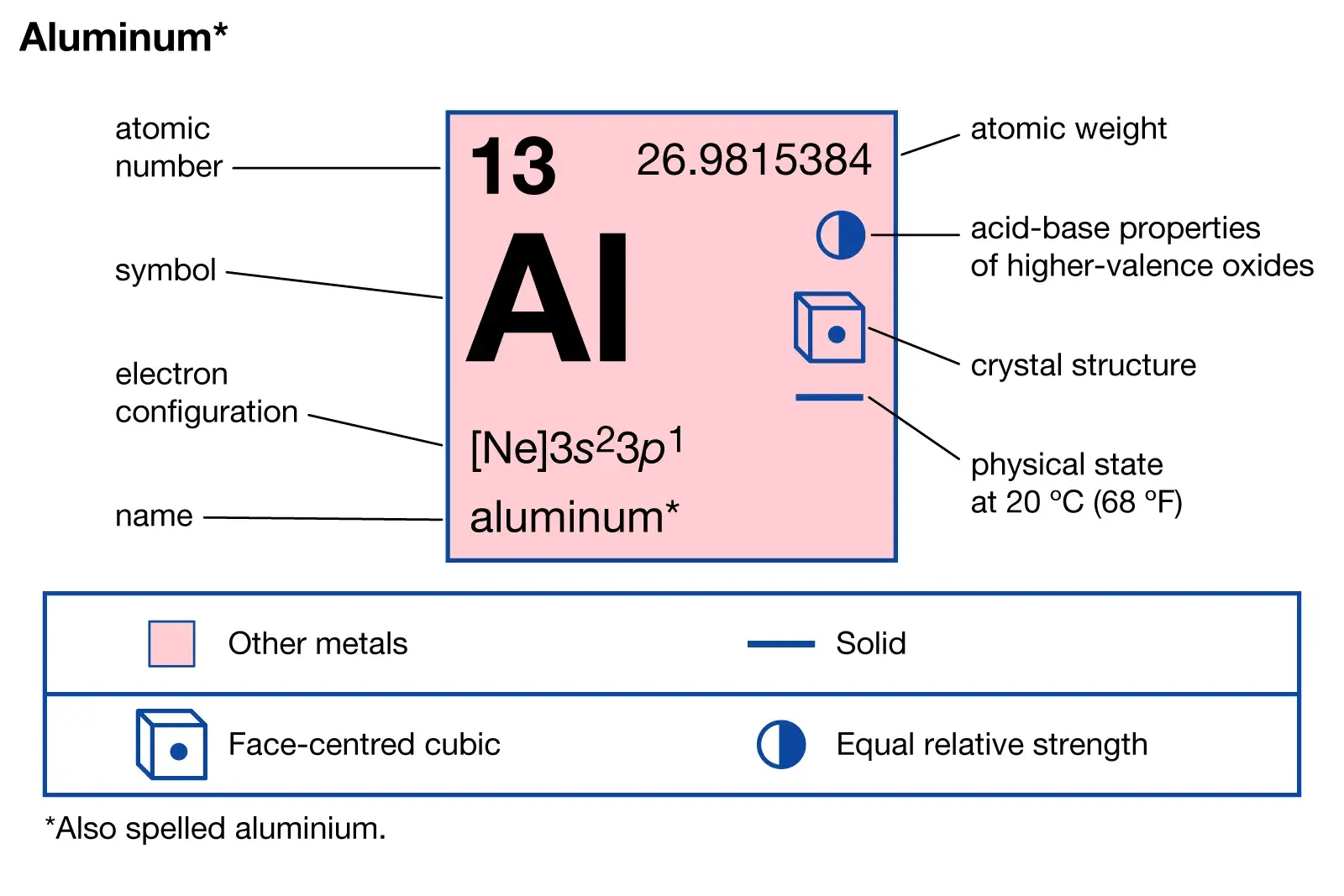
Aluminum is a popular metal in manufacturing due to its lightweight and strength-to-weight ratio. Combining aluminum with elements like copper, magnesium, silicon, and zinc creates aluminum alloys with unique properties for various industries. These alloys are used in consumer electronics, packaging, and parts for automobiles and airplanes. This article will explain the definition of aluminum alloys and their properties, categories, characteristics, and uses.

Table of Contents
ToggleAluminum is a versatile metal that can be combined with other elements to alter its properties. For example, they combine magnesium with aluminum, producing a strong, lightweight alloy ideal for aerospace and automotive industries. Aluminum alloys have extensive use in many fields due to their low density, resistance to corrosion, and excellent thermal conductivity. They are used to produce various items such as consumer electronics, automobiles, and aircraft.
During the early stages of research and development, aluminum alloys were primarily associated with the aviation industry, which had a history of only nearly a century. However, they rapidly developed, and their range of applications continued to expand. Among metal materials, their production ranks second after steel and first among non-ferrous metal materials. Aluminum alloys are expected to develop significantly by adopting updated process methods, improving existing alloys, developing new alloys, endowing them with updated and more comprehensive performance, and expanding the scope of usage.
In 1908, Alcoa Inc. invented electrical aluminum alloy 1050 and made it into steel core aluminum stranded wire, pioneering high-voltage remote transmission.
In 1915, Alcoa Inc. invented the 2017 alloy; in 1933, it invented the 2024 alloy, rapidly expanding the application of aluminum in aircraft. In 1933, the American Aluminum Company invented 6061 alloys and immediately created the aluminum extrusion quenching process, significantly expanding the application range of extruded profiles.
In 1943, Alcoa Inc. invented 6063 alloys and 7075 alloys, ushering in a new era of high-strength aluminum alloys.
1965, Alcoa invented a classic cast aluminum alloy, documented as number 1.
With the deepening of research on aluminum alloy materials, high-strength aluminum alloys (2000, 7000 series) in commercial aircraft have reached over 80% of their structural mass due to their excellent comprehensive performance, thus receiving widespread attention from the global aviation industry. Aluminum alloy is gradually being applied in daily life and in the military and technological fields.
The following are some common elements present in aluminum alloys:
Copper (Cu): Adding copper to aluminum enhances strength and hardness. An example of an aluminum-copper alloy is 2024.
Zinc (Zn): Aluminum-zinc alloys are recognized for their durability and resistance against corrosion. A well-known example of this type of alloy is 7075.
Magnesium (Mg): Aluminum-magnesium alloys are light in weight and possess excellent corrosion resistance. An example of such an alloy is 5083.
Silicon (Si): Incorporating silicon into aluminum enhances its casting properties and reduces shrinkage while solidifying. The 4043 aluminum alloy is one such alloy that offers such distinct qualities.
It is essential to remember that numerous metallic and non-metallic elements can be added to aluminum alloys to improve their properties further. For instance, chromium can enhance corrosion resistance, while magnesium can be added to increase strength and toughness. Because of this, a vast array of aluminum alloys is readily available, each with a unique set of properties and potential uses.
Chemical Composition Limit Of Aluminum Alloys | |||||||||||
| Aluminum Alloys | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Others | Aluminum | |
| Each | Total | Min. | |||||||||
| 2024 | 23.2 | 0.5 | 3.8-4.9 | 0.3-0.9 | 1.2-1.8 | 0.1 | 0.25 | 0.15 | 0.05 | 0.15 | Remain |
| 5052 | 25 | 0.4 | 0.1 | 0.1 | 2.2-2.8 | 0.15-0.35 | 0.1 | — | 0.05 | 0.15 | Remain |
| 5083 | 23.8 | 0.4 | 0.1 | 0.3-1.0 | 4.0-4.9 | 0.05-0.25 | 0.25 | 0.15 | 0.05 | 0.15 | Remain |
| 6061 | 23.6 | 0.7 | 0.15-0.4 | 0.15 | 0.8-1.2 | 0.04-0.35 | 0.25 | 0.15 | 0.05 | 0.15 | Remain |
| 7050 | 23.5 | 0.15 | 20.-2.6 | 0.1 | 1.9-2.6 | 0.04 | 5.7-6.7 | 0.06 | 0.05 | 0.15 | Remain |
| 7075 | 23.6 | 0.5 | 1.2-2.0 | 0.3 | 2.1-2.9 | 0.18-0.28 | 5.1-6.1 | 0.2 | 0.05 | 0.15 | Remain |
Typical Mechanical Properties of Aluminum Alloys | ||||
| Aluminum Alloys and Temper | Tensile Strength(25°C MPa) | Yield Strength(25°C MPa) | Hardness 500kg Force 10mm Ball | Elongation 1.6mm (1/16in) thickness |
| 5052-H112 | 175 | 195 | 60 | 12 |
| 5083-H112 | 180 | 211 | 65 | 14 |
| 6061-T651 | 310 | 276 | 95 | 12 |
| 7050-T7451 | 510 | 455 | 135 | 10 |
| 7075-T651 | 572 | 503 | 150 | 11 |
| 2024-T351 | 470 | 325 | 120 | 20 |
Typical Physical Properties of Aluminum Alloys | |||||
| Aluminum Alloys and Temper | Thermal Expansion | Melting Point | Electrical Conductivity20℃(68℉) | Resistivity20℃(68℉) | Density(20℃)(g/cm3) |
| (20-100℃) | (℃) | (%IACS) | Ωmm2/m | ||
| μm/m·k | |||||
| 2024-T351 | 23.2 | 500-635 | 30 | 0.058 | 2.82 |
| 5052-H112 | 23.8 | 607-650 | 35 | 0.05 | 2.72 |
| 5083-H112 | 23.4 | 570-640 | 29 | 0.059 | 2.72 |
| 6061-T651 | 23.6 | 580-650 | 43 | 0.04 | 2.73 |
| 7050-T7451 | 23.5 | 490-630 | 41 | 0.0415 | 2.82 |
| 7075-T651 | 23.6 | 475-635 | 33 | 0.0515 | 2.82 |

Alloys are metals composed of a combination of other metals or non-metallic elements. They may be commonly known by a name or identified using a four-digit number, with the first digit indicating the series of alloys.
The 1xxx series alloys are made of 99% or higher purity aluminum, also known as commercially pure aluminum.
The 2xxx series has copper as its principal alloying element. Heat treating these alloys can enhance their strength but are not as corrosion-resistant as other aluminum alloys. Therefore, they are typically painted or powder-coated before use. One of the hardest aluminum alloys is 2024-T351, the most common aircraft alloy.
The 3xxx series of aluminum alloys primarily contains manganese and a smaller amount of magnesium. Among these alloys, 3003 is the most commonly used, as it is both workable and moderately strong. It finds its application in the manufacturing of cooking utensils. Alloy 3004, on the other hand, is one of the alloys used to make aluminum cans for beverages.
The 4xxx series alloys contain silicon, which is added to aluminum to reduce the melting point of the metal without making it brittle. These alloys are used to make welding wire. Alloy 4043, in particular, makes filler alloys for welding cars and structural elements.
The 5xxx series alloys have magnesium as their principal alloying element. They are strong, weldable, and resistant to marine corrosion, which makes them ideal for pressure vessels, storage tanks, and marine applications. For instance, alloy 5182 is used to make the lid of aluminum beverage cans. Therefore, aluminum cans consist of at least two alloys.
The 6xxx series alloys have silicon and magnesium, which combine to form magnesium silicide. These alloys are formable, weldable, and heat-treatable, with moderate strength and good corrosion resistance. The 6061 alloy is widely used in manufacturing truck and boat frames. Additionally, extrusions products from the 6xxx series are used in architecture to make the iPhone 6.
The series starting with the number 7 has zinc as its principal alloying element. The resulting alloy is heat-treatable and very strong. Some important alloys in this series are 7050 and 7075, used to construct aircraft.
The 8xxx series alloys are made with other elements, such as 8500, 8510, and 8520.
The series starting with the number 9 is currently unavailable.

Aluminum alloys possess excellent characteristics such as low density, high strength, resistance to corrosion, and good formability, which make them useful across multiple industries. The following are a few popular uses of aluminum alloys:
Vehicle manufacturers use different aluminum forged parts to create lightweight, durable, and environmentally friendly cars that are agile and rugged due to aluminum alloys’ high durability and lightweight properties.
Aluminum alloy is the primary material used for manufacturing airplanes. Compared to the soft steel used in automobiles, aluminum alloy is more expensive and has a lower density, with a relative density 2.8. It is about one-third lighter than soft steel, with a relative density of 7.8, even though the strength difference is insignificant. Lightweight materials are crucial for airplanes, requiring strong corrosion resistance and ease of processing. As a result, aluminum alloy is the most suitable material for airplane manufacturing.
By varying its alloy element content, hard aluminum can produce rivets, aircraft propellers, and high-strength parts of the airplane. Super hard aluminum is a type of hard aluminum that contains zinc, making it harder and stronger than hard aluminum. Different types of super-hard aluminum are utilized to manufacture various structural and high-load parts, making it one of the essential materials in the aviation industry.
Aluminum alloys are often used in consumer goods due to their minimal weight and ability to resist corrosion. Automobile parts, aluminum tubing parts, cookware, aluminum sheet, aluminum pipe and beverage cans are just a few examples of products that are usually made of aluminum. Durability, strength, and recyclability are advantages of using an aluminum alloy in consumer goods.
Aluminum alloy is used in various equipment in the medical industry, such as wheelchairs, hospital beds, and surgical instruments, due to its low density, relative strength, and corrosion resistance. It is also utilized in medical implants like bone plates and screws because it is biocompatible. It’s very often crucial for a medical device to be durable and immune to rust and other types of corrosion.
Aluminum alloy is an excellent material for metal packaging due to its various advantageous characteristics. It has good mechanical properties, is lightweight, possesses high compressive strength, is durable, and makes storing and transporting goods easy. Additionally, it has a good barrier performance, which can effectively block the damage of sunlight, oxygen, and humid environments to items, thereby extending the shelf life of products. Moreover, aluminum alloy has a unique metallic luster, which gives it a good texture and aesthetic appeal, contributing to improved product quality. Lastly, it is non-toxic and easy to recycle, saving resources and reducing environmental pollution.
Beer, beverages, and other food cans mostly use aluminum alloy in stamping and drawing structures. Aluminum foil containers are lightweight and beautiful, with good heat transfer properties. They are used for packaging fast food and have the effects of preservation, flavor preservation, and non-toxicity, making them increasingly popular in the food industry. Aluminum alloy metal hoses can be extruded and deformed, and the contents can be used by squeezing, making them simple and convenient for packaging cream cosmetics.

Aluminum alloys offer various advantages, including:
Aluminum alloys are perfect for harsh environments such as marine or industrial applications because they offer excellent corrosion resistance.
Aluminum alloys are ideal for applications where reducing weight is crucial. Compared to other metals, aluminum items are much lighter in weight.
Aluminum alloys are stronger than many other materials that have a similar density.
Most aluminum alloys are highly ductile, so they can be easily shaped without breaking or cracking.
Aluminum alloys make good thermal conductors, which makes them suitable for use in applications where heat needs to be transferred efficiently.
Some limitations of aluminum alloys include:
Aluminum is relatively soft compared to certain other materials, so it may not withstand as much wear.
Aluminum alloys have a lower melting point than most structural metals. It makes them unsuitable for high-temperature applications.
In specific scenarios, aluminum alloys might not be as economical as other materials.

On October 27th, 2017, the International Agency for Research on Cancer of the World Health Organization released a preliminary list of carcinogens, and the production process of aluminum was listed as a Class I carcinogen.
Studies have shown that aluminum can damage the cells in the human brain. According to the World Health Organization, the recommended daily intake of aluminum is 0-0.6mg/kg, meaning that a 60kg person is allowed to consume 36mg daily.
Aluminum slowly accumulates in the body, causing slow and almost undetectable toxicity. However, if metabolic disorders occur, the consequences can be severe. Therefore, it is essential to prevent aluminum absorption and reduce the daily use of aluminum products. The harm caused by aluminum and its compounds to humans cannot be compared to their benefits. Being cautious and avoiding weaknesses can be more critical in human society.
Here are some methods that can help to avoid aluminum exposure:
1. Avoid using aluminum cookware.
2. Reduce the consumption of foods such as fried dough sticks, candy packed with aluminum, and soft drinks in cans.
3. Some drugs are made from substances that contain aluminum, and the consumption of such medicines should be reduced. For example, Al (OH) 3 can neutralize stomach acid.

The article introduces all aspects of aluminum alloys and their properties, categories, characteristics, and applications. Enze offers various aluminum CNC machining services and aluminum sheet metal fabrication of different parts. If you need a quote, contact us.

