Our Location
Copper is a versatile metal with excellent conductivity and thermal conductivity. It is widely used in manufacturing cables, brushes, and other products that require good conductivity. It is also used in producing conductive and thermal conductive equipment. Copper is known for its good plasticity, which makes it easy to process by hot and cold pressure.
Copper has good resistance to corrosion in various environments, such as the atmosphere, seawater, certain nonoxidizing acids, salt solutions, and different organic acids, making it a valuable material in the chemical industry.
Table of Contents
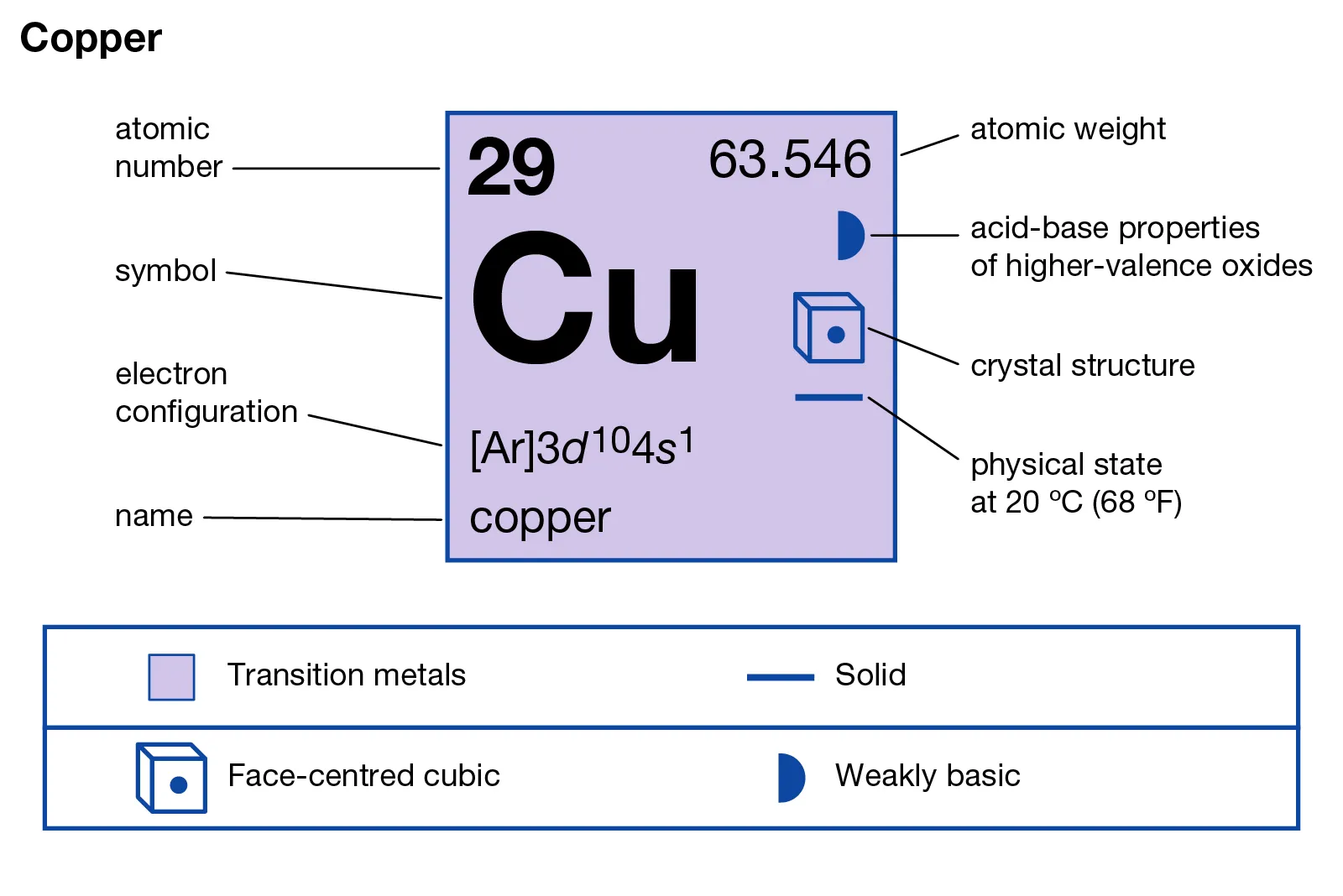
ToggleCopper is a pure chemical element with the symbol Cu and atomic number 29 used in industry. It is known for its reddish-brown color that appears after an oxide film forms on its surface. This element is commonly referred to as copper but also red copper. When copper contains a certain amount of oxygen, it is called oxygen-containing copper and can sometimes be considered a copper alloy.
Copper, which is symbolized as Cu on the periodic table of elements, is also known by its Latin name cuprum. The name cuprum originated from the island of Cyprus, a major copper source in ancient times.
Source: Britannica
The history of copper dates back to 8,700 BC, when a copper pendant was found in Northern Iraq. This pendant is believed to be the oldest object made of copper. Copper was a vital metal in many ancient civilizations, and it is widely agreed that the Mesopotamians, who lived in Northern Iraq, were the first to discover it. The period between 5,500 BC and 4,000 BC is known as the Chalcolithic Age or Copper Age, derived from the Greek words for copper (chalkos) and rock (lithos).
Copper is one of the few materials that can occur naturally in its native metallic form. It differs from most metals, which need to be extracted through metallurgy from an ore. For those civilizations that had access to native copper, there was no need for metallurgy to work with copper and create weapons.

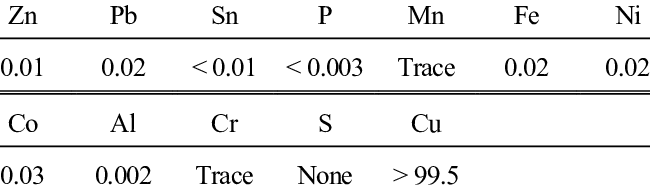
It contains copper and silver as the main components, with a 99.7-99.95% content. The main impurity elements in purple copper are phosphorus, bismuth, antimony, arsenic, iron, nickel, lead, tin, sulfur, zinc, oxygen, and others.
Copper has good conductivity and plasticity but has relatively poor strength and hardness. It has excellent thermal conductivity, ductility, and corrosion resistance. However, the trace impurities in copper can significantly impact its conductivity and thermal conductivity. Titanium, phosphorus, iron, silicon, and other elements can substantially reduce conductivity, while cadmium, zinc, and others have little effect.
The solubility of sulfur, selenium, tellurium, and other elements in copper is very low, and they can form brittle compounds with copper. It can reduce processing plasticity, but it has little effect on conductivity.
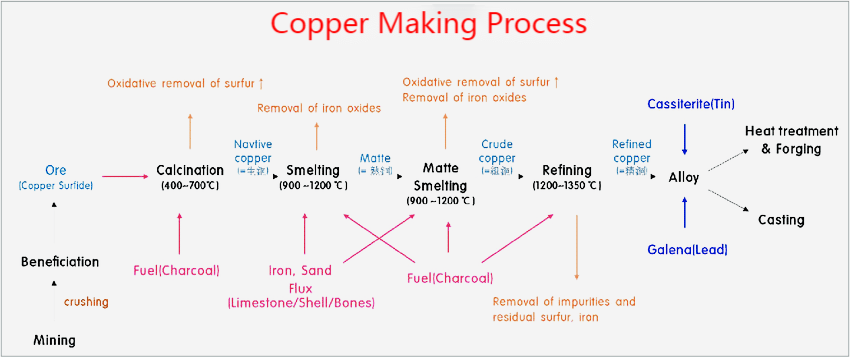
The process of making copper involves different stages as below:
The initial step in the copper process involves the selection of ores that meet quality requirements. Preferable ores for copper extraction include those with high copper content, such as hematite and sphalerite.
There are two main purification processes for copper ores, depending on whether they are oxide or sulfide ores. For oxide ores, a hydrometallurgical process involves heaping the crushed ore and percolating an acid-leaching solution to create a pregnant leach solution. For sulfide ores, a pyrometallurgical method is used, which consists of extracting the ore by froth flotation and thickening based on the density of the particles.
The pregnant leach solution is concentrated in copper through a solvent extraction process for oxide ores. This solution is then sent to electrowinning, where electricity is used to deposit the solid copper. A smelter creates the raw copper for sulfide ores, which is further purified by electrorefining.
Copper alloys are made by melting the alloying material first, then melting the copper and adding it to the mixture. The molten mixture is then cast and allowed to cool and solidify.
Electrorefining involves electrolytically dissolving impure copper material into a solution and then electrochemically depositing pure copper on an electrode by applying an electrical current. It removes impurities from the copper to achieve higher purity. However, the process is expensive and has a very high electrical demand.
Copper is a metal with excellent electrical and thermal conductivity, malleability, and ductility. It is resistant to corrosion and aesthetically pleasing with its reddish-brown color. Pure copper is pink before oxidation but quickly forms a brown oxide layer, giving it its characteristic color. Copper can also have a green tarnish, like the Statue of Liberty, a copper carbonate. Copper looks like a shiny, reddish-brown metal and is used in various forms, such as wires, pipes, and the automotive industry.
Metals have different grades based on their purity levels. Impurities introduced during the composition process can affect their properties and fabricability. Copper can be refined in various ways to achieve desired purity levels. These are the commercial grades of pure copper:
These are of the purest quality, with a minimum of 99.99% copper, and have the lowest level of volatile impurities. They are produced via induction melting, where a high-quality cathode copper is placed into a granulated graphite bath, creating a nonoxidizing and low-hydrogen atmosphere. This type of copper is highly conductive and is commonly used in high-vacuum electronics, such as glass-to-metal seals and transmitter tubes. Its UNS designation ranges from C10100 to C10200.

These contain 0.7% impurities in their composition. Its softness and ductility characterize it. To increase its stiffness, other elements are often added. The amount and type of these additional alloys and additives will vary, resulting in diluted coppers with a UNS number ranging from C10100 to C13000, based on the added elements and impurity levels.

Copper refined using electrolysis creates various products. During electrolysis, copper compounds are placed into a solution, and electricity is applied to purify the copper metal. Electrolytic copper is highly conductive and ductile with an electrical conductivity of 100-101% IACS and is widely used in electrical applications. It contains less than 50 ppm of metallic impurities, can be worked with hot and cold processes, and has the UNS number C11000.
Free-machining coppers contain minor amounts of other elements (<1%) to enhance machinability. They are commonly used to produce welding nozzles and soldering iron tips.
Understanding the differences between these grades could help you select copper that offers the appropriate thermal and electrical conductivity levels while being cost-effective for your projects.

Copper is a pure material with high purity levels, almost pure copper. It has good conductivity and plasticity, but its strength and hardness could be improved. Copper has excellent thermal conductivity and corrosion resistance. However, even small amounts of impurities in copper can seriously affect its conductivity and thermal conductivity. Elements like titanium, phosphorus, iron, and silicon can significantly reduce conductivity, while cadmium and zinc have little effect. The solubility of sulfur, selenium, tellurium, and other elements in copper is very low, and they can form brittle compounds with copper, reducing its processing plasticity. However, they have little effect on conductivity.
Copper has excellent resistance to corrosion in various environments, including the atmosphere, seawater, and certain nonoxidizing acids like hydrochloric acid and dilute sulfuric acid. It is also resistant to alkalis, salt solutions, and other organic acids like acetic and citric acids. It makes copper a popular choice for use in the chemical industry. Additionally, copper is highly weldable and can be easily shaped into semi-finished and finished products using cold and hot plastic processing methods.
Impurities in copper, such as titanium, can significantly impact its ability to conduct heat and electricity. Cadmium and zinc, on the other hand, have little effect on copper’s conductivity. Elements like oxygen don’t dissolve easily in copper and can create brittle compounds that reduce plasticity during processing, although they have minimal conductivity effects.
When heated with hydrogen or carbon monoxide, copper can react with Cu2O at grain boundaries, causing high-pressure water vapor or carbon dioxide gas to form, which can rupture copper. Oxygen harms copper’s weldability, while bismuth or lead causes copper to become thermally brittle by forming a low melting point eutectic with copper. Bismuth, as a thin film at grain boundaries, can cause copper to become cold and brittle.
Phosphorus can significantly reduce the conductivity of copper, but it can improve copper’s liquid fluidity and weldability. Moderate amounts of lead, tellurium, sulfur, and other elements can improve machinability. Annealed copper sheet has a tensile strength of 22-25 kgf/mm2 at room temperature, an elongation of 45-50%, and a Brinell hardness (HB) of 35-45. Finally, pure copper has a 386.4 W/(m·K) thermal conductivity.
The following are three crucial material property parameters for copper:
1. Elastic modulus: This measures the rigidity of a material and its ability to undergo elastic deformation under stress. It is expressed as E, and its elastic modulus is approximately 100-110 GPa for copper.
2. Shear modulus: This refers to the ratio of the reaction force and stress generated by the deformation of a material under shear stress. It plays a significant role in material mechanics. For copper, its shear modulus is approximately 45-50 GPa.
3. Poisson’s ratio: This ratio describes a material’s proportional expansion or contraction under isotropic conditions in two directions perpendicular to the direction under pressure in one direction. The Poisson’s ratio ranges between 0 and 0.5, and its value is approximately 0.33 for copper.
| Properties of Copper | |||
| Young’s Modulus | Shear Modulus | Poisson ratio | Hardness(HV) |
| 110-128 MPa | 48GPa | 0.34 | 343-369 MPa |
Copper is used to make various welding parts and can be welded using different methods. Below are the three most common ways:

For this method, you can use either wire 201 or wire 202 welding wire with flux 301. Before welding, it is recommended to preheat the materials at a temperature range between 400-700℃. Use a neutral flame with higher flame power during welding, and reduce welding stress by using fewer layers and hammering the weld seam after welding.
To weld copper using this method, you can use copper 107 or copper 227 welding rods. The power supply should adopt a DC reverse connection. Before welding:
You can use the same wire and flux as in oxygen acetylene welding. The power supply should adopt a DC-positive connection. Before welding, preheat the materials, but the temperature should not be too high.
Copper has a broader range of uses compared to pure iron. Every year, 50% of copper is electrolyzed and purified into pure copper used in the electrical industry. The copper used in this industry must be pure, with a copper content of over 99.95%. Even minimal impurities, like phosphorus, arsenic, aluminum, etc., can significantly reduce copper’s conductivity. It is primarily used to make electrical equipment such as generators, cables, busbars, transformers, switchgear, and thermal conductivity equipment such as flat plate collectors for heat exchangers, pipelines, and solar heating devices. The oxygen content in copper, which is easily mixed with oxygen during copper smelting, significantly impacts conductivity, and copper used in the electrical industry generally must be oxygen-free.
In order to prevent heat embrittlement and facilitate pure copper processing, impurities such as lead, antimony, and bismuth must be removed. High-purity copper is usually refined using a process called electrolysis. In this process, impure copper acts as the anode, while pure copper is the cathode. Copper sulfate solution is used as the electrolyte. As the current passes, impure copper on the anode gradually melts, while pure copper slowly precipitates on the cathode. Copper is refined through this process, enabling it to achieve a purity level of up to 99.99%.
Copper has several advantages as listed below:
1. Good thermal conductivity, used in heat exchangers such as refrigeration and air conditioning.
2. Excellent electrical conductivity; it is the widely used conductor for electronic and electrical components on a global scale.
3. Corrosion-resistant, particularly in water and seawater environments. It has led to its extensive use in plumbing pipes for water systems.
4. Antimicrobial, which can be very beneficial for water conveyance and in medical applications to safeguard patients’ health.
5. Ductile and can be bent with relative ease. It is simple to work with in plumbing systems, as well as in cabling.
Limitations of copper are as follows:
1. Heavy, making it less practical for overhead wires.
2. Expensive, compared to cheaper materials like aluminum or plastics.
3. Oxidizes, particularly at high temperatures, which limits its usage lifespan when exposed to air.
4. Toxic, so it cannot be used in utensils or other objects that come into regular contact with food or drink.
5. Copper presents a risk of electric shock compared to alternative signal transmission technologies, such as fiber optics.
Copper does not rust because rust is an iron oxide, and copper has no iron. However, copper can experience some minor surface corrosion. Copper is generally considered to be corrosion-resistant. It has a natural reddish-brown color due to the formation of a copper oxide passivation film on the metal’s surface. As time passes, a green copper carbonate forms on the surface as the copper oxide reacts with carbon dioxide and water in the air.
Copper undergoes oxidation when it reacts with oxygen, blackening over time. So, it’s recommended to perform surface treatment after processing.
Yes, copper can be poisonous to humans in significant concentrations. Copper is an essential trace mineral for human health, but several complications arise if an excess of copper is present. A high blood concentration of copper is linked to Alzheimer’s disease and particular types of cancers such as breast and lung cancer. Acute copper poisoning is very rare but can occur if large amounts are absorbed. It can be fatal as failure of organs such as the liver and kidneys begins to happen.
1. Brass is a metal alloy composed of copper and zinc. It may also contain smaller amounts of tin, lead, aluminum, or manganese to achieve specific properties.
2. Copper has a reddish-brown appearance; brass is yellowish-gold.
3. To determine whether a material is a copper or a metal alloy, you can lightly strike it against a surface. Copper will produce a low and deep sound, while Brass will generate a higher sound than copper.
4. Brass is best for machining, while copper is the most flexible option.
This article overviews copper’s history, definition, types, and applications. You want to learn more about copper, you can contact the Enze representative. Enze offers a variety of manufacturing capabilities to meet your prototyping and production requirements.

